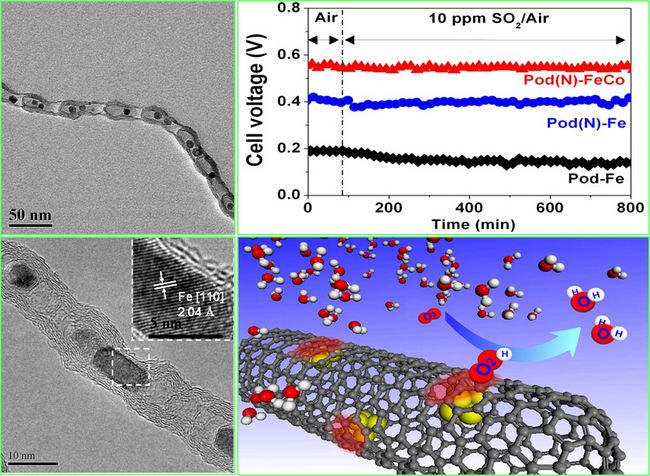
The direct and efficient conversion of hydrogen into electrical energy that can be widely used, while generating water molecules that are friendly to human survival, is an important goal for the development of advanced sustainable energy systems in the future. In order to achieve this goal, the proton exchange membrane fuel cell as an important energy conversion device will play an irreplaceable role, and related research and development have received more and more attention. However, the cathode materials used in this type of fuel cell to efficiently reduce oxygen molecules in the air to active oxygen atoms and the anode materials that catalyze the dissociation of hydrogen molecules require large amounts of precious metals, such as platinum, palladium, ruthenium, etc., as catalysts. It will become an important bottleneck for the future large-scale application of fuel cells. Therefore, greatly reducing the content of precious metals in fuel cell electrode materials, and eventually replacing precious metals with earth's abundant "cheap" metal elements have become major opportunities and challenges in this field, and related research has formed fierce competition around the world Situation. Existing research results indicate that metallic iron and low-valent compounds with unsaturated coordination iron have excellent catalytic oxygen reduction (ORR) characteristics. However, due to the active low-valent iron atoms in the catalytic process, they are extremely susceptible to excessive oxidation. The iron oxides (Fe2O3) that are complex and saturated are rapidly eroded in the acidic environment where the fuel cell operates, causing the battery electrode to quickly lose catalytic activity. Dr. Deng Dehui, State Key Laboratory of Catalysis Basics, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dr. Pan Xiulian, Academician Bao Xinhe, and others cooperated with the Fuel Cell Research Department of the National Laboratory of Clean Energy to build a series of progress in the study of the limit effect of carbon nanotubes. Efforts to innovate synthetic preparation methods, creatively confine iron-based metal nanoparticles into the lumen of carbon nanotubes with bean-pod-like structures (Bean-Pod-Like), using the newly developed deep ultraviolet light emission electrons newly developed by the research group Microscope (DU-PEEM), and with the help of Shanghai X-ray advanced X-ray absorption spectroscopy (XAS), combined with theoretical calculations, for the first time observed that the active d electrons of metallic iron "pass through through interaction with the carbon atoms that make up the carbon tube wall" "(Penetrating) carbon tube wall, electrons enriched on the outer surface of the carbon tube directly catalyze the reduction reaction (ORR) of molecular oxygen. Experimental and theoretical studies further confirmed that in this system, the carbon wall encapsulating the nano-metallic iron blocks the direct contact between the reaction gas and the iron nanoparticles, and in principle avoids the deep oxidation of the active metal iron nanoparticles during the reaction And the poisoning of the catalyst by other harmful components in the reaction atmosphere, thus fundamentally solving the problem of the stability of nano-metallic iron as a cathode catalyst for fuel cells. Further doping hetero-atoms (such as nitrogen atoms) on the carbon wall of the metal nanoparticles and changing the composition of the metal nanoparticles in the metal nanoparticles can significantly modulate the catalytic performance of the electrode material. When the carbon nanotube wall nitrogen doping concentration is 3.3 wt% and the Fe-Co nanoalloy is wrapped, the power density of the battery can reach 60% of the 20% Pt-C catalyst under the exact same operating conditions. In particular, in the presence of 10 ppm of the harmful component sulfur (SO2), the battery still maintains excellent activity and stability. This result was recently published online by "German Applied Chemistry". This research not only provides an effective way to study the precious metal substitution of fuel cell catalysts, but also develops into the concept of "Chainmail for catalyst" for catalysts that will operate under harsh conditions in the future. The design and preparation of the vehicle opened up new directions. The paper has been selected as an important development and will be officially published in a special issue to be published in the first issue of 2013 to celebrate the 125th anniversary of the publication of the German Applied Chemistry. The above research has been supported by related projects such as the National Natural Science Foundation of China and the Ministry of Science and Technology. Dalian Chemical Research Institute made important breakthroughs in the research of precious metal substitution of fuel cell catalysts
Single Fabric Sofa is a recliner sofa with one seat. It's cover materials is fabric which is safe, green and environmentally friendly, comfortable cloth, made of wood frame and fine sponge, which brings people warm, comfortable and environmentally friendly family living environment.
Fabric sofa is not as stiff as solid wood sofa, you`ll feel very comfortable when you sit on it, and breathable performance of fabric sofa is good. The soft cloth is especially comfortable to touch, making people relax and feel the leisure.
Fabric sofa is suitable for small space, it makes more sofa designs and colors than leather sofa, and it`s warm in winter, and breathable in summer, new fabric sofa won't give out a pungent leather smell at the same time.
Fabric sofa gives a person a sense of leisure, and strong comfort, let a person have the feeling of relax when get home.
Single Fabric Sofa,Power Reclining Sofa Chair,Power Lift Massage Sofa,Home Massage Recliner Sofa Kaifeng Lanwei Smart Home Co., Ltd , https://www.leather-recliner.com